MORE DETAILS ABOUT THE FUNCTION OF THE MEMBRANE
MORE DETAILS ABOUT THE FUNCTION OF THE MEMBRANE

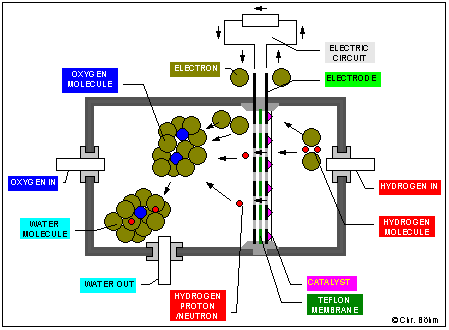
The hydrogen and the oxygen burns "cold" (40°C - 90°C) in a fuel cell to form water. The fuel cell consists of a membrane which is only permeable forh the protons of the hydrogen, but the hydrogen-molecule is very stable. A catalyst is required to divide the hydrogen molecule into the proton which passes the teflon-membrane and the electron, which has to pass the electric circuit to reach the other side of the membrane. There the electrons and the hydrogen-protons react with the oxygen-molecule to form a water-molecule. But the reaction is only possible when the catalyst has a minimum temperature of about 40°C.
2H2 + O2 = 2H2O
The reactions in the fuel cell are reversible. When a electric power-supply is connected to the both electrodes and you inject water in the fuel cell, the cell will produce oxgen gas and hydrogen gas. The gases can be stored to burn them later.